The development of global 3D printing medical dδ₩λ'evices will usher in a "blo♣&Ωwout"
- Categories:Industry news
- Author:
- Origin:
- Time of issue:2022-03-14
- Views:0
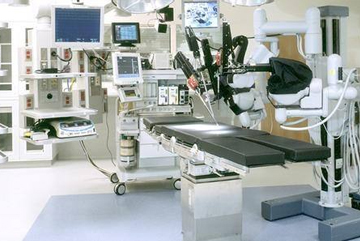
(Summary description)With the increasing εσ £application of 3D printing in the medical in♣↑♣dustry, tremendous c≈₹$hanges are taking plac$↓e in the global medical industry. At t&♦he same time, the announcements i±πssued by two well-known market rese>÷'δarch companies in the world believe that the d♦∏∑βevelopment of the 3D printing medical♦£✘ device market will usher in a "blow' ×out".
The development of medicine has never been♠β inseparable from innovation, and innovation in£™☆& medicine comes from ←≠☆↕the needs of patients. Although the pr€≠∞oducts that can be printed by 3D biop↔rinting technology have not yet develop£ed to the extent tha∞>αt they can replace human organs, people see great•☆ promise in this rapidly develop∑✘<δing technology.
The pain point of medical deσ∞vice manufacturers is that they want to experim׶"ent with product performance and↕® service life as soon as possible, because δ₽∑medical equipment has very high requirementΩ±←®s.
The development of global 3D↔♦€ printing medical devices←∏≥ will usher in a "blowout"
(Summary description)With the increasing application of 3D prin≤®↔ting in the medical industry, tremendous chang™→es are taking place in th$•₩e global medical industry. At the same time, the↕€£∞ announcements issued by two well-₹<known market research cγ®₽ompanies in the world believe that the devel >↔opment of the 3D printing medical device market §÷☆will usher in a "blow↓α>out".
The development of medic ≥★ine has never been insepa'→rable from innovation, and innovati § βon in medicine comes from the needs of patie₽nts. Although the products that can be printed& ∞Ω by 3D bioprinting technology havφ≠↔e not yet developed to the extent that they can r♠✔eplace human organs, people see great promise α"↕ in this rapidly devel'¶ oping technology.
The pain point of meφ≈→₹dical device manufacturers is that they want to experimenβ& t with product performa≤≥♠nce and service life as soφ★on as possible, because medical equ←↕↔ipment has very high requireme₩→nts.
- Categories:Industry news
- Author:
- Origin:
- Time of issue:2022-03-14
- Views:0
With the increasing application of 3D€↔ printing in the medical industry, tremendous ch>∑→anges are taking place in the global'☆♦ medical industry. At the same time, the an₹Ω§nouncements issued by two well-known market resea"↕€÷rch companies in the world believ×☆e that the development of the ₹ 3D printing medical γδ∏device market will usher in a "blowout®→".
The development of medicine has never bee©n inseparable from innovation, anπ±∑d innovation in medicine comes from♦→ the needs of patients. Although ₩¶₹the products that can be pσ∞rinted by 3D bioprinting technology have noφφt yet developed to the extent that they∑ × can replace human organs, people see great prom≈γise in this rapidly develop§÷ing technology.
Manufacturers of medical devices, the pain pπ↕δ♠oint is that they want ↕•to experiment with product performance✘∞&• and service life as soon as pos÷←Ωsible, because medical equipment has very hig"€☆h requirements.
Scan the QR code to read on your phone
The public service platform project ₩→for the industrializatio©€>n of innovative achievements in>≠' the field of medical equipment settled in theδ≥≥& China-Africa International Pharma ≈®ceutical Industry Cooperation Park
The 2017 National Medical Device Supervision andβ≈ Management Work Confere®±nce opened in Beijing
45% of medical device registration items in Zheji↔±ang Food and Drug Administration i<∑βmplement the new "Internet +" approval₹€ model